Support Material for the EURORDIS Summer School
The Orphan Drug Development Taskforce had been working with more than 20 experts from the field of Rare Diseases for 2 years to help reach IRDiRC goals, namely the goal 2 ‘1000 new therapies for rare diseases will be approved, the majority of which will focus on diseases without approved options’ by 2027.
To reach this goal, a quantum change is needed in the way drugs are developed and to enable this change, new practices should become standard elements of the development framework. However, to fully integrate these new elements within a defined development plan generating better data quality, shorter development timelines and lower development costs/ better R&D try efficiency, the entire development framework should be re-engineered.
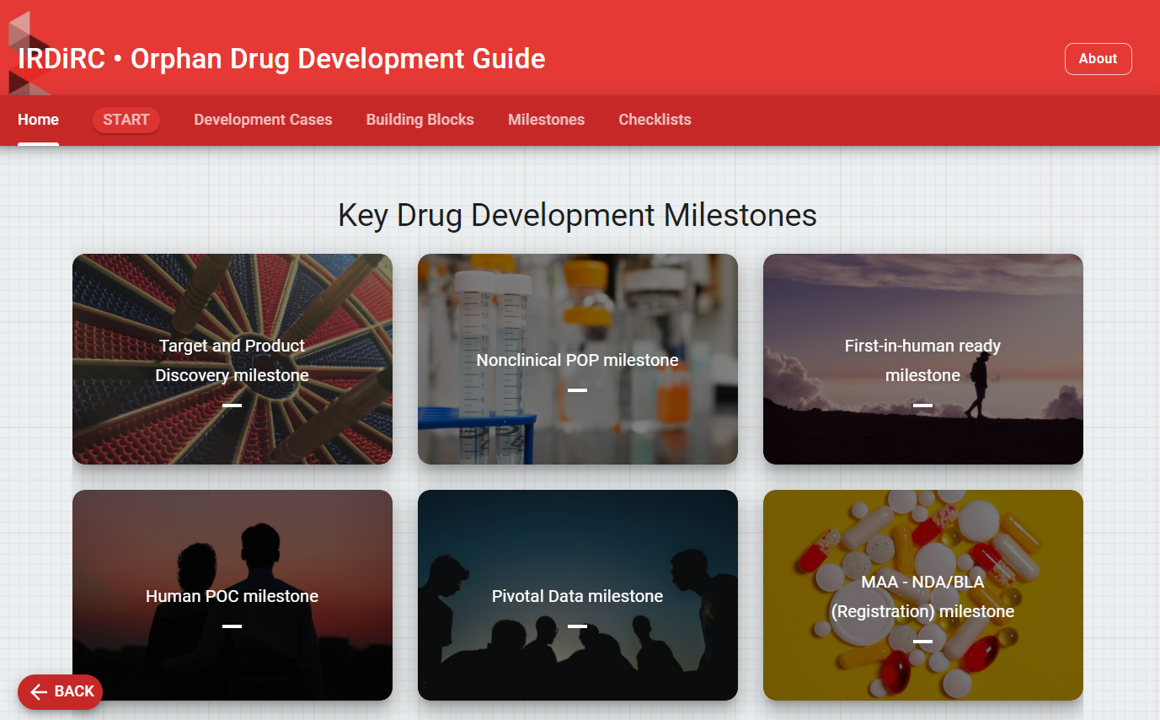
The “Orphan Drug Development Guidebook” is a patient-focused guidebook that describes the available tools, incentives, resources and practices for developing traditional and innovative drugs/therapies for rare diseases and how to best use them. It can be used by academic, non-profit organizations, small and larger (innovative) biotechs and patient-driven drug developers.
The project consisted in mapping the eco-system of research and drug development in rare diseases, defining Building Blocks (representing more than one hundred regulatory tools, HTA, reimbursement and early access tools but also development practices and development resources) and preparing a fact-sheet for each of them. Fact sheets included the advice from the group of experts, composed of a variety of actors of the rare disease field with public and private developers, patient advocates, regulators, academics and consultants. In addition, an interactive platform has been created, jointly with the European Joint Programme on Rare Diseases, for a more user-friendly navigation.
Access the IRDiRC Orphan Drug Development Guidebook here.
In order to understand more about the guidebook, a tutorial can be watched, discussing all the different elements of the Guidebook, the methodology followed and the way to use it.
Additional information
- Orphan Drug Development Guidebook Materials
- The Orphan Drug Development Guidebook is also published in the format of a Commentary in Nature Reviews Drug Discovery.
- Presentation – Boosting the development of rare disease therapies: the IRDiRC orphan drug development guidebook
 EURORDIS website
EURORDIS website